BRUKINSA patients can call the myBeOne Support™ program to talk to a dedicated nurse: 1-833-234-4363
SEQUOIA AND ALPINE: 2 GLOBAL PHASE 3 TRIALS IN CLL
A global Phase 3, randomized, open-label, multicenter trial conducted in patients with previously untreated CLL/SLL.
Trial was initiated in 2017.*
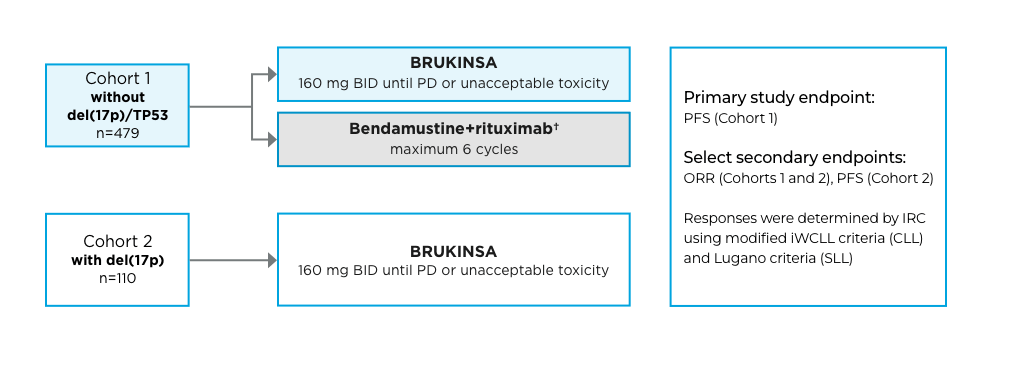
Cohort 2 rationale: Because patients with CLL/SLL whose tumors exhibit del(17p) have an unfavorable prognosis and respond poorly to standard chemoimmunotherapy, those with del(17p) mutations were assigned to receive BRUKINSA in this separate single-arm exploratory analysis.
*The primary endpoint was PFS per IRC in the ITT population in the BRUKINSA arm and the BR arm, with minimum 2-sided alpha of 0.05 for superiority.2
†Patients received bendamustine 90 mg/m2/day on the first 2 days of each cycle for 6 cycles, rituximab 375 mg/m2 for Cycle 1, and rituximab 500 mg/m2 for Cycles 2-6.1
1L=first line; BID=twice daily; BR=bendamustine+rituximab; CLL=chronic lymphocytic leukemia; IRC=independent review committee; ITT=intent to treat; iWCLL=International Workshop on Chronic Lymphocytic Leukemia; ORR=overall response rate; PD=progressive disease; PFS=progression-free survival; SLL=small lymphocytic lymphoma.
BASELINE PATIENT CHARACTERISTICS IN A RANGE OF PATIENTS, INCLUDING THOSE WITHOUT DEL(17p)/TP53 AND WITH DEL(17p) MUTATIONSPatient characteristics1-3
| Baseline Patient Characteristics | Cohort 1 Patients Without Del(17p)/TP53 | Cohort 2 Patients With Del(17p) | |
|---|---|---|---|
| BRUKINSA (n=241) | BR (n=238) | BRUKINSA (n=111) | |
| Median age, years | 70 (IQR: 66-75) | 70 (IQR: 66-74) | 70 (IQR: 66-74) |
| Age ≥65 years | 81% | 81% | 86% |
| Male | 64% | 61% | 71% |
| Caucasian | 92% | 87% | 95% |
| ECOG PS 2 | 6% | 8% | 13% |
| Binet stage C | 29% | 29% | 35% |
| Target lesion ≥5 cm | 29% | 31% | 40% |
| Unmutated IgHV, n/N (%) | 125/234 (53%) | 121/231 (52%) | 67/103 (60%) |
| Del(17p) | 1% | 0% | 99% |
| Del(11q) | 18% | 19% | 33% |
| TP53 mutation | 6% | 6% | 43% |
| Complex karyotype status | |||
| <3 abnormalities | 35% | 33% | 49% |
| ≥3 abnormalities | 8% | 5% | 29% |
| ≤5 abnormalities | 40% | 36% | 57% |
| ≥5 abnormalities | 3% | 1% | 21% |
| Samples not yet evaluated | 58% | 63% | 23% |
Comorbidities3
| Baseline Patient Characteristics | Cohort 1 Patients Without Del(17p)/TP53 | Cohort 2 Patients With Del(17p) | |
|---|---|---|---|
| BRUKINSA (n=241) | BR (n=238) | BRUKINSA (n=111) | |
| History of cardiac disorders* | 27% | 29% | 29% |
| History of afib/flutter | 5% | 11% | 7% |
| Hypertension | 56% | 55% | 51% |
*Cardiac disorders is a grouped term of 33 conditions that includes atrial fibrillation, myocardial ischemia, coronary artery disease, myocardial infarction, cardiac arrest, cardiac failure, acute coronary syndrome, angina pectoris, tachycardia, and bradycardia.2,3
Afib=atrial fibrillation; BR=bendamustine+rituximab; ECOG PS=Eastern Cooperative Oncology Group performance status; IQR=interquartile range.
A global Phase 3, randomized, open-label, multicenter trial conducted in patients with relapsed/refractory CLL/SLL who received ≥1 prior systemic therapy.*

*Statistical analysis for PFS and ORR was initially conducted for noninferiority. When noninferiority was met, superiority was tested. Assessments were made by the investigators, with similar results by IRC.
2L=second line; afib=atrial fibrillation; BID=twice daily; CLL=chronic lymphocytic leukemia; IRC=independent review committee; iWCLL=International Workshop on Chronic Lymphocytic Leukemia; ORR=overall response rate; PD=progressive disease; PFS=progression-free survival; QD=once daily; SLL=small lymphocytic lymphoma.
BRUKINSA WAS STUDIED IN A RANGE OF PATIENTS, INCLUDING THOSE WITH AND WITHOUT MUTATIONS ASSOCIATED WITH POOR PROGNOSISPatient characteristics3,4
| Baseline Patient Characteristics* | BRUKINSA (n=327) | Ibrutinib (n=325) |
|---|---|---|
| Median age, years | 67 (range: 35-90) | 68 (range: 35-89) |
| Age ≥65 years | 61% | 62% |
| Male | 65% | 71% |
| Caucasian | 80% | 82% |
| ECOG PS 2 | 2% | 4% |
| Binet stage C | 44% | 42% |
| Median prior lines of therapy | 1 (range: 1-6) | 1 (range: 1-12) |
| Prior treatments | ||
| Anti-CD20 antibody | 84% | 83% |
| Alkylators (other than bendamustine) | 84% | 80% |
| Purine analogue | 54% | 52% |
| Bendamustine | 26% | 29% |
| PI3K/SYK inhibitor | 3% | 6% |
| BCL2 inhibitor | 2% | 3% |
| iMiD | 2% | 0.3% |
| Alemtuzumab | 0.6% | 0.3% |
| Chemoimmunotherapy | 80% | 76% |
| Del(17p) deleted/abnormal | 14% | 15% |
| Umutated IgHV | 73% | 74% |
| Del(11q) deleted/abnormal | 28% | 27% |
| TP53 mutation | 9% | 8% |
| Complex karyotype status† | ||
| Yes | 17% | 22% |
| No | 47% | 40% |
| Missing | 36% | 39% |
Comorbidities3
| Baseline Patient Characteristics* | BRUKINSA (n=327) | Ibrutinib (n=325) |
|---|---|---|
| History of cardiac disorders‡ | 28% | 29% |
| History of afib/flutter | 6% | 6% |
| Hypertension | 49% | 48% |
*Values based on patients enrolled in the ITT population.3
†Complex karyotype is defined as having 3 or more abnormalities.3
‡Cardiac disorders is a grouped term that includes atrial fibrillation, myocardial ischemia, coronary artery disease, myocardial infarction, arrhythmia, atrial flutter, cardiac failure, tachycardia, bradycardia, and cardiac aneurysm.3
Afib=atrial fibrillation; ECOG PS=Eastern Cooperative Oncology Group performance status; iMiD=immunomodulatory drug; ITT=intent to treat.
IMPORTANT SAFETY INFORMATION
What should I tell my healthcare provider before taking BRUKINSA?
Before taking BRUKINSA, tell your healthcare provider about all of your medical conditions, including if you:
- have bleeding problems.
- have had recent surgery or plan to have surgery. Your healthcare provider may stop BRUKINSA for any planned medical, surgical, or dental procedure.
- have an infection.
- have or had heart rhythm problems.
- have high blood pressure.
- have liver problems, including a history of hepatitis B virus (HBV) infection.
- are pregnant or plan to become pregnant. BRUKINSA can harm your unborn baby. If you are able to become pregnant, your healthcare provider may do a pregnancy test before starting treatment with BRUKINSA.
- Females should avoid getting pregnant during treatment and for 1 week after the last dose of BRUKINSA. You should use effective birth control (contraception) during treatment and for 1 week after the last dose of BRUKINSA.
- Males should avoid getting female partners pregnant during treatment and for 1 week after the last dose of BRUKINSA. You should use effective birth control (contraception) during treatment and for 1 week after the last dose of BRUKINSA.
- are breastfeeding or plan to breastfeed. It is not known if BRUKINSA passes into your breast milk. Do not breastfeed during treatment with BRUKINSA and for 2 weeks after the last dose of BRUKINSA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking BRUKINSA with certain other medications may affect how BRUKINSA works and can cause side effects.
What are the possible side effects of BRUKINSA?
BRUKINSA may cause serious side effects, including:
- Bleeding problems (hemorrhage). Bleeding problems are common with BRUKINSA, and can be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs or symptoms of bleeding, including:
- blood in your stools or black stools (looks like tar)
- pink or brown urine
- unexpected bleeding, or bleeding that is severe or you cannot control
- vomit blood or vomit that looks like coffee grounds
- cough up blood or blood clots
- increased bruising
- dizziness
- weakness
- confusion
- change in speech
- headache that lasts a long time
- Infections that can be serious and may lead to death. Tell your healthcare provider right away if you have fever, chills, or flu-like symptoms.
- Decrease in blood cell counts (white blood cells, platelets, and red blood cells). Your healthcare provider should do blood tests during treatment with BRUKINSA to check your blood counts.
- Second primary cancers. New cancers have happened in people during treatment with BRUKINSA, including cancers of the skin or other organs. Your healthcare provider will check you for other cancers during treatment with BRUKINSA. Use sun protection when you are outside in sunlight.
- Heart rhythm problems (atrial fibrillation, atrial flutter, and ventricular arrhythmias) that can be serious and may lead to death. Tell your healthcare provider if you have any of the following signs or symptoms:
- your heartbeat is fast or irregular
- feel lightheaded or dizzy
- pass out (faint)
- shortness of breath
- chest discomfort
- Liver problems. Liver problems, which may be severe or life-threatening, or lead to death, can happen in people treated with BRUKINSA. Your healthcare provider will do blood tests to check your liver before and during treatment with BRUKINSA. Tell your healthcare provider or get medical help right away if you have any signs of liver problems, including stomach pain or discomfort, dark-colored urine, or yellow skin and eyes.
The most common side effects of BRUKINSA include:
- decreased white blood cell count
- decreased platelet count
- upper respiratory tract infection
- bleeding
- muscle, bone, or joint pain
These are not all the possible side effects of BRUKINSA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is BRUKINSA?
BRUKINSA is a prescription medicine used to treat adults with:
- Chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
- Waldenström’s macroglobulinemia (WM).
- Mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer.
- Marginal zone lymphoma (MZL) when the disease has come back or did not respond to treatment and who have received at least one certain type of treatment.
- Follicular lymphoma (FL), in combination with the medicine obinutuzumab, when the disease has come back or did not respond to treatment and who have received at least two prior treatments.
It is not known if BRUKINSA is safe and effective in children.
Please see full Prescribing Information including Patient Information.

