BRUKINSA is a BTK inhibitor that was designed to block BTK
- BRUKINSA has been shown to block 100% of BTK in blood cells and 94% to 100% of BTK in lymph nodes when taken at the recommended total daily dose of 320 mg. The significance of blocking up to 100% of BTK on treatment responses has not been established
Why is a BTK inhibitor important for follicular lymphoma (FL) treatment?
FL is caused by the rapid growth and spread of cancerous B cells.
- Bruton’s tyrosine kinase (BTK) is a protein that signals within cancerous B cells, helping them to grow and spread
- Blocking BTK can help stop this signaling
How well does BRUKINSA work?
BRUKINSA was shown to be effective with obinutuzumab in the ROSEWOOD study. Obinutuzumab is a type of treatment called a monoclonal antibody (mAB) that is used to treat different types of lymphomas. The aim of the study was to see if BRUKINSA + obinutuzumab was more effective than obinutuzumab alone.
The study included 217 patients with FL who had received at least 2 prior treatments. In the ROSEWOOD study, different types of responses were studied. Overall response rate (ORR), or the percentage of patients in which treatment is working, was the main purpose of the study. Additional responses studied were duration of response (DOR), or the amount of time before treatment stopped working, and progression-free survival (PFS), or the length of time which patients lived without their disease worsening.
BRUKINSA is the first and only BTK inhibitor approved for the treatment of FL
In the ROSEWOOD study, significantly more patients were proven to respond to BRUKINSA + obinutuzumab than obinutuzumab alone.
At 20 months, more patients taking BRUKINSA + obinutuzumab saw an improvement* in their FL compared to patients taking obinutuzumab alone
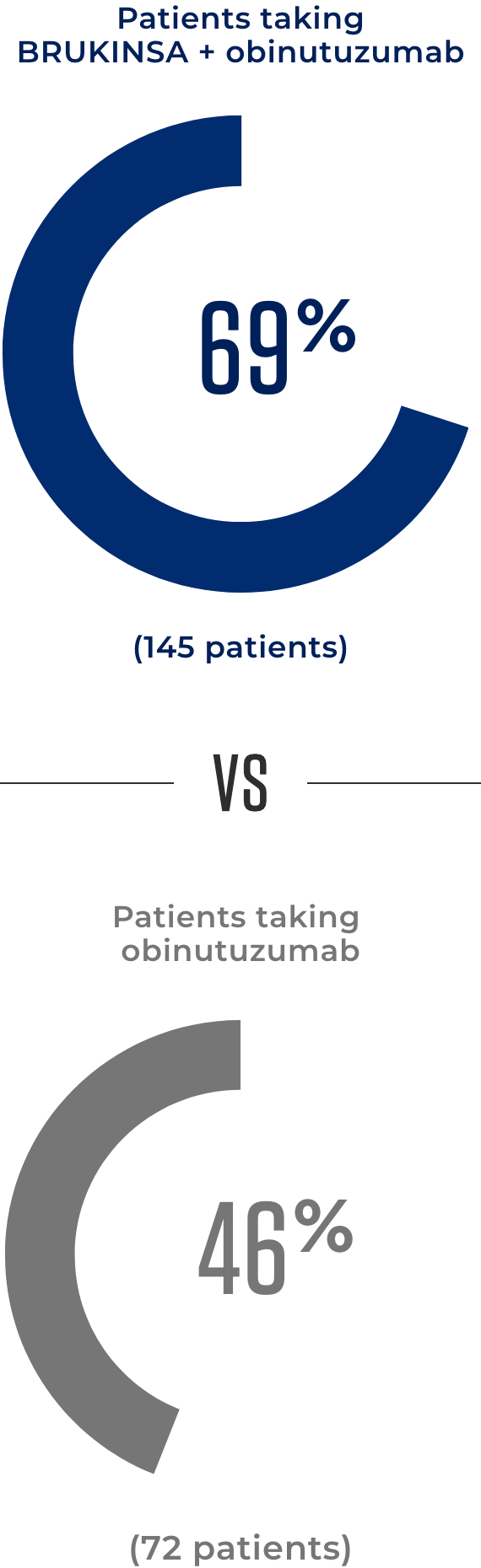
*When patients improved, tests showed that their cancer got smaller in size and did not spread.
At 18 months, treatment continued working for more patients taking BRUKINSA + obinutuzumab than obinutuzumab alone
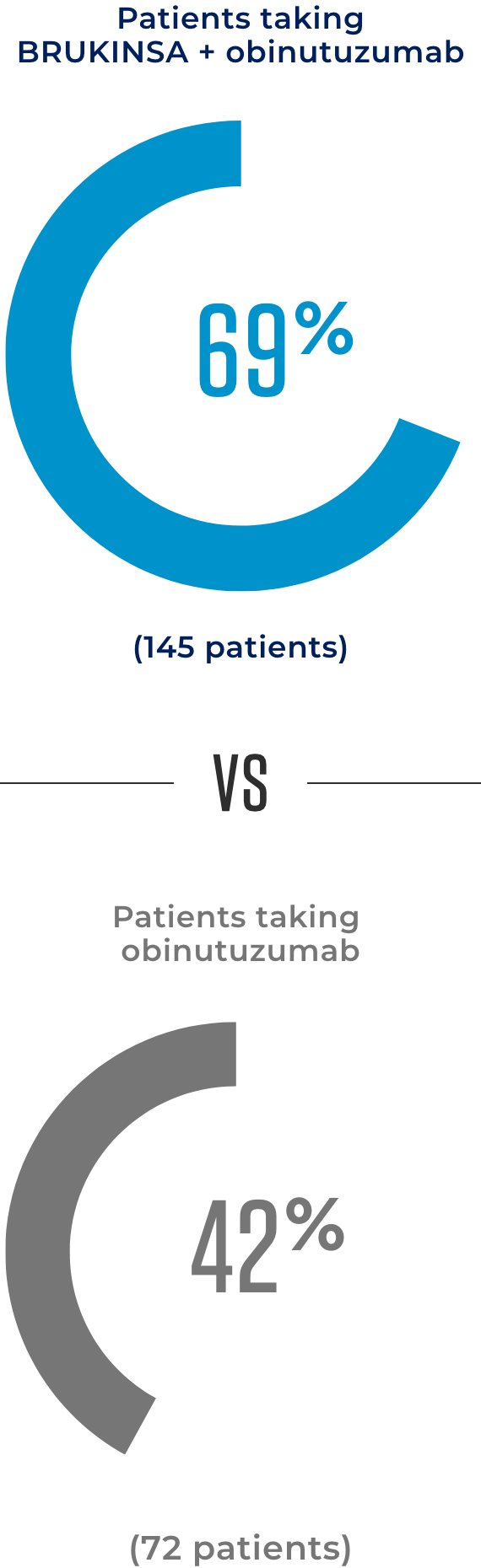
The amount of time in which the median, or 50% of patients in each treatment group, continued to see no worsening of their FL
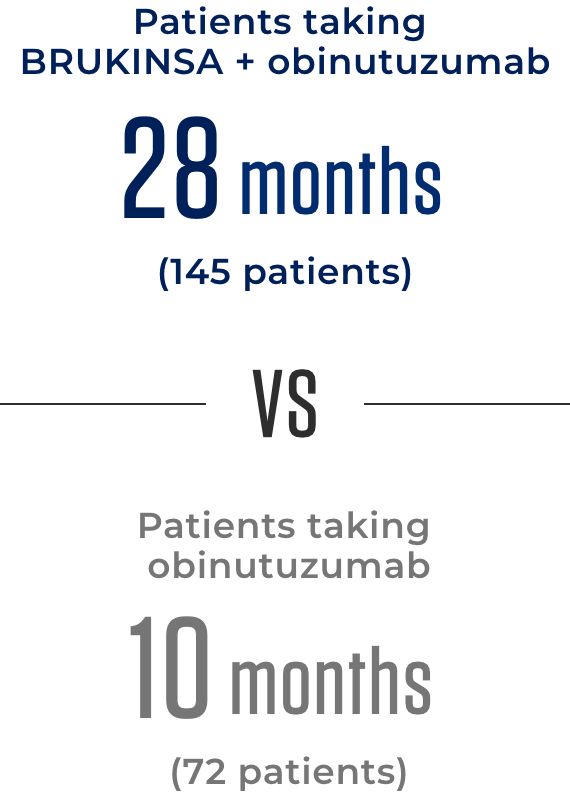
This suggests that the combination of BRUKINSA + obinutuzumab may delay worsening of FL by as much as 18 months.
These results presented are consistent with, but not included in, the FDA-approved prescribing information.
Individual results may vary.