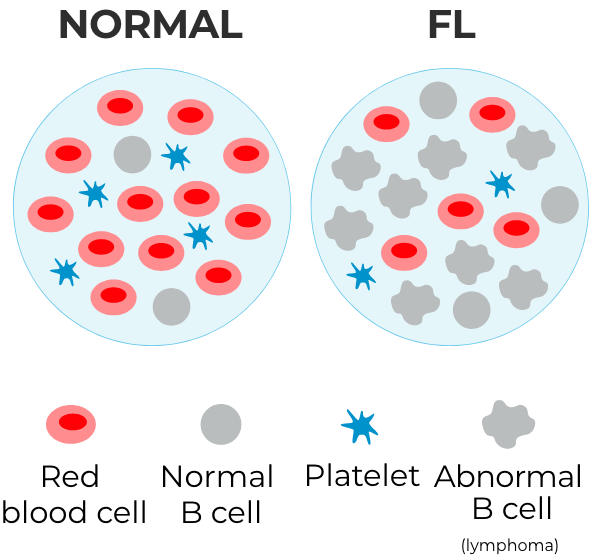
The National Comprehensive Cancer Network® (NCCN®) recommends zanubrutinib (BRUKINSA®), in combination with obinutuzumab, as an NCCN Category 2A treatment option for people with FL who have received at least two prior treatments.*
NCCN is a not-for-profit alliance of 33 leading cancer centers in the United States devoted to patient care, research, and education. The organization creates cancer guidelines that are considered the standard to guide treatment decisions.
* Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 18, 2025. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.